
The first FDA approved treatment for Neuroendocrine tumors
Introducing LUTATHERA® (lutetium Lu 177 dotatate), the first FDA and HSA approved Peptide Receptor Radionuclide Therapy (PRRT) for treating Neuroendocrine tumors is now proudly offered by Parkway Radiology.
The PRRT treatment with lutetium-177 dotatate (Lu-177) is now available at Mount Elizabeth Orchard and Mount Elizabeth Novena Hospital. Such treatment requires the integrated efforts of a multidisciplinary team and unique clinical settings.
What are Neuroendocrine tumors (GEP-NETs)
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are tumors derived from neuroendocrine cells that can occur anywhere along the gastrointestinal tract and comprise of a mixed group of neoplasms (abnormal mass of tissue) with a wide and complex spectrum of clinical behavior.
GEP-NETs have commonly been divided into foregut, midgut and hindgut tumors.
What is Lutetium-177 Dotatate (Lu-177) and how does it treat
Lutetium-177 dotatate (Lu-177) is a prescription treatment for adults with a type of cancer known as GEP-NETs that have somatostatin hormone receptors.
The treatment comprises of a 2-part approach that specifically targets and enters the cells that have somatostatin receptors, releasing energy in the form of radiation that damages them and nearby cells.
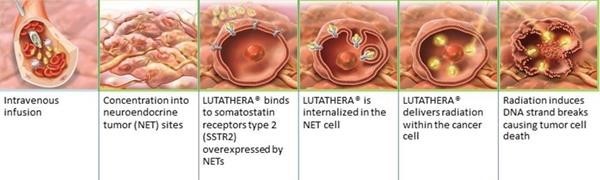
Method of action for PRRT with Lutathera®. Credit: Advanced Accelerator Applications (AAA)
Understanding the Lutetium-177 Dotatate (Lu-177) Treatment Journey
1. Seeking Consultation from Healthcare Professionals
Patients should always first seek consultation from a healthcare professional to assess the suitability of taking on this treatment. It’s important to share everything about your medical condition, health status, current medications, pregnancy plans etc.
2. Before Starting First Treatment with Lutetium-177 Dotatate (Lu-177)
An imaging test will be performed to locate the tumor. Patient may also have to undergo some blood tests and other tests for evaluation. Patient may also be injected with a medicated drug as needed for symptom management.
3. Day of Treatment
Patient will be given medicines before the infusion for managing side effects and protection of kidneys.
Lutetium-177 dotatate (Lu-177) is administered by insertion through slow intravenous (IV) infusion over a 30-40 minute time span. As the treatment uses radiation, you will be asked to wait a while before leaving the treatment premises.
The treatment cycle repeats up to 4 times at 8-week intervals.
You may Contact us for any further details or enquiries.
Sources:
- Advanced Accelerator Applications (AAA)
- Parkway Radiology
- National Library of Medicine
Updated 03 Nov 2022